CMS Vaccine Mandate Rule Update
- Sammy Chang
- Jan 4, 2022
- 7 min read
In the Midst of the Fog of Litigation, CMS Begins Enforcement of Vaccine Mandate for Half the Country
On December 28, 2021, Centers for Medicare & Medicaid Services (“CMS”) announced that it would begin enforcement of its vaccine mandate as set forth in its November 5, 2021 Interim Final Rule (hereinafter known as the “CMS Vaccine Mandate”). As part of its announcement, CMS issued a new memorandum outlining the new deadlines and requirements for compliance under the vaccine mandate. With respect to hospitals, rural health clinics (“RHC”), and federally qualified health centers (“FQHCs”) (referenced here as “facilities”), CMS’s new memorandum is summarized below in three parts:
A. What are the New Deadlines to Comply with the CMS Vaccine Mandate?
B. Who Must Be Vaccinated under the CMS Vaccine Mandate?
C. What are the Policies and Procedures that Must be Implemented?
CMS made clear that enforcement only applies to the 25 states(1) that have not sued to prohibit the implementation of the vaccine mandate.
In the meantime, litigation continues regarding the vaccine mandate. The U.S. Supreme Court will hear arguments regarding both the CMS vaccine mandate and the OSHA vaccine mandate on January 7, 2022. However, as it is unclear how the U.S. Supreme Court will decide on the CMS vaccine mandate, facilities should work toward complying with the CMS vaccine mandate.

A. What are the New Deadlines to Comply with the CMS Vaccine Mandate?
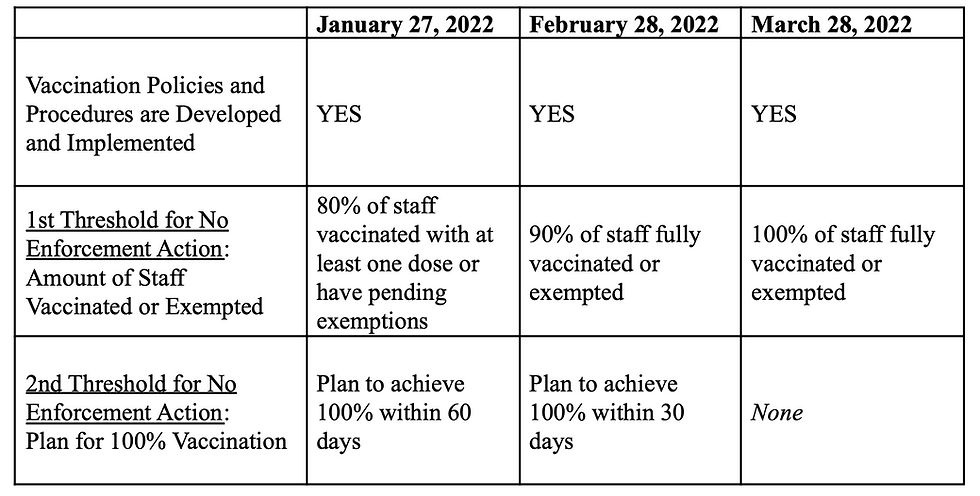
CMS’s December 28, 2021 memorandum delineated new deadlines for facilities to comply with the vaccine mandate as described below. CMS recognized it would be difficult for facilities to achieve 100% vaccination within 30 or 60 days and, therefore, articulated two thresholds for the January 27, 2022 and the February 28, 2022 deadlines that a facility must achieve to avoid enforcement actions. Both thresholds must be reached in order to avoid an enforcement action.
CMS also indicated it would lower the level of the enforcement action if the facility showed a good faith effort to obtain vaccine access (when the facility has limited or no access to vaccines) or taken aggressive steps to have all staff vaccinated, such as advertising for new staff and hosting vaccine clinics. However, by March 28, 2022, failure to reach 100% vaccination AND develop and implement one or more components of the policies and procedures will most likely result in an enforcement action.
January 27, 2022 – To be in full compliance, a facility must have:
All required policies and procedures are developed and implemented
100% of staff have:
Received at least one dose of the vaccine OR
Pending request for exemption OR
Been granted a qualifying exemption OR
Been identified as having a temporary delay as recommended by the CDC
However, for the January 27, 2022 deadline, facilities would not be subject to enforcement action by CMS if the facility is at 80% of the above AND has a plan to achieve a 100% staff vaccination rate within 60 days.
February 28, 2022 – To be in full compliance, a facility must have:
All required policies and procedures are developed and implemented
100% of staff have:
Received the necessary doses to complete the vaccine series OR
Been granted a qualifying exemption OR
Been identified as having a temporary delay as recommended by the CDC
However, for the February 28, 2022 deadline, facilities would not be subject to enforcement action by CMS if the facility is 90% of the above AND has a plan to achieve a 100% staff vaccination rate within 30 days.
March 28, 2022 – Facilities are subject to enforcement action if it does not reach or maintain the “100% standard” and have all required policies and procedures developed and implemented.
TABLE 1: Summary of Deadlines and Enforcement Thresholds Per December 28, 2021 Guidance:
B. Who Must Be Vaccinated under the CMS Vaccine Mandate?
In addition to the preamble language in the November 5th rule, the December 28 memorandum provided language regarding which staff members are required to be vaccinated. The scope of the vaccination mandate is summarized below.
Vaccination is required for:
Individuals who provide any care, treatment, or other services for the facility and/or its patients, which includes facility employees; licensed practitioners; students, trainees, and volunteers; and individuals under contract or under an arrangement providing services on a regular basis – regardless of frequency of patient contact.
Examples of individuals under contract or under an arrangement includes mental health professionals, social workers, and portable x-ray suppliers.
CMS specifically identified that the following individuals would have to be vaccinated: administrative staff, facility leadership, volunteer or other fiduciary board members, and housekeeping and food services.
CMS was clear that vaccination is required for any staff who has direct contact with staff who are required to be vaccinated OR uses the same common areas used by staff, patients, and visitors. To be clear, CMS explained that this includes staff who have the potential to have contact with staff or patients at the site of care. For example, vaccination requirements extend to staff who primarily provide services remotely via telework BUT occasionally encounter fellow staff, such as in an administrative office or at an off-site staff meeting, who will themselves enter a health care facility or site of care for their job responsibilities.
2. No vaccination is needed for:
Individuals who very infrequently provide ad hoc non-healthcare services (such as annual elevator inspection, delivery, and repair that are not under contract or arrangement)
Individuals who provide services 100 percent remotely or exclusively off-site, such as fully remote telehealth or payroll or accounting services
Staff who exclusively provide telehealth or telemedicine services outside of the facility setting and who do not have any direct contact with patients and individuals who are required to be vaccinated (as indicated above)
Staff who provide support services for the hospital that are performed exclusively outside of the facility setting and who do not have any direct contact with patients or individuals who are required to be vaccinated (as indicated above)
Note that CMS requires that these staff members must be identified and monitored, including the documentation of overall vaccine status.
C. What are the Policies and Procedures that Must be Implemented?
Pursuant to the December 28 memorandum, CMS directed surveyors to determine whether the facility implemented the following processes and policies by January 27, 2022. For ease of reading, the required policies and procedures are grouped into four categories.
Vaccination Compliance:
Policies and procedures for ensuring all staff specified in Section B.1:
Have received the single-dose or first dose of COVID-19 vaccine prior to staff providing any care, treatment, or other services for the facility and/or its patients.
Have received any recommended booster doses, and any recommended additional doses for individuals who are immunocompromised, in accordance with the recommended timing of such doses
Are fully vaccinated for COVID-19
This process would exclude any staff:
Who have been granted exemptions to the vaccination requirements
Whose COVID-19 vaccination must be temporarily delayed, as recommended by the CDC, due to clinical precautions and considerations.
2. Vaccination Exemptions:
Policies and procedures by which staff may request an exemption from the staff COVID-19 vaccination requirements, which should include:
How an exemption can be requested, including for those arising from the Americans with Disabilities Act and Title VII of the Civil Rights Act of 1964
To whom the request must be made
Collection of requests
Evaluation of requests:
For religious exemptions, CMS directs facilities to the Equal Employment Opportunity Commission (EEOC) Compliance Manual on Religious Discrimination for further guidance, (https://www.eeoc.gov/laws/guidance/section-12-religious-discrimination)
Accommodations for an unvaccinated employee with a qualifying exemption
For medical exemptions, policies and procedures for ensuring that documentation, which confirms recognized clinical contraindications to COVID-19 vaccines and which supports staff requests for medical exemptions from vaccination are:
Signed and dated by a licensed practitioner
The policies must include the requirement that the licensed practitioner cannot be the individual requesting the exemption and is acting within their respective scope of practice as defined by, and in accordance with, all applicable State and local laws.
Specifying which of the authorized COVID-19 vaccines are clinically contraindicated for the staff member to receive and the recognized clinical reasons for the contraindications
Including a statement by the authenticating practitioner recommending that the staff member be exempted from the facility’s COVID-19 vaccination requirements for staff based on the recognized clinical contraindications
3. Mitigation of COVID-19 and Additional Precautions for Not Fully Vaccinated Staff:
Policies and procedures to ensure that the facilities follow nationally recognized infection prevention and control guidelines intended to mitigate the transmission and spread of COVID-19.
A policy to educate on facility’s policies and procedures for unvaccinated staff and offer vaccination to all unvaccinated staff. There is no requirement to counsel or educate staff on COVID-19 vaccination.
Policies and procedures to implement additional precautions or accommodations for all staff who are not fully vaccinated for COVID-19
Examples of additional precautions include:
Re-assignment or modification of staff duties to teleworking or duties that limit exposure to patients at most risk of COVID-19.
CDC-recommended precautions like physical distancing
Weekly testing
Use a NIOSH-approved N95 or equivalent or higher-level respirator for source control
Contingency plans for staff who have not completed the primary vaccination series for COVID-19, which may include the additional precautions listed above as well as the following:
Staff that are not fully vaccinated, including those who have pending exemption requests, do no qualify for exemption, or are temporarily delayed in their vaccination will not provide care, treatment, or other services for the provider or its patients until such time as such staff, at a minimum, received the first dose of COVID–19 vaccine or is granted an exemption.
Deadline for each unvaccinated staff to have received their first dose of a vaccine
Actions facility will take if deadline is not met, such as:
Actively seeking replacement staff through advertising or
Obtaining temporary vaccinated staff until permanent vaccinated replacements can be found
4. Secure Tracking of Vaccination Status or Exemption:
Policies and procedures for tracking and securely documenting:
The COVID-19 vaccination status of all staff (including the specific vaccine received, and the dates of each dose received (including boosters), or the date of the next scheduled dose, if applicable);
The vaccination status of staff for whom COVID-19 vaccination must be temporarily delayed, as recommended by the CDC, due to clinical precautions and considerations AND when the identified staff can safely resume their vaccination;
Information provided by those staff who have requested, and for whom the facility has granted, an exemption from the staff COVID-19 vaccination requirements (including type of exemption and supporting documentation);
Information confirming recognized clinical contraindications to COVID-19 vaccines provided by those staff who have requested and have been granted a medical exemption to vaccination; and
Accommodations that are granted with respect to a vaccine exemption; and
For unvaccinated individuals only, education of the facility’s policy and procedure regarding unvaccinated individuals
Policies and procedures to identify:
Percentage of staff not vaccinated or granted an exemption
Each staff’s role, assigned work area, and how they interact with patients (including those of contractors’, volunteers’, and students’)
Newly hired staff (hired in the last 60 days)
Staff who telework full-time (e.g., 100 percent of their time is remote from sites of patient care and staff who do work at sites of care
Please note CMS is explicit that all medical records, including vaccine documentation, must be kept confidential and stored separately from an employer’s personnel files, pursuant to ADA and the Rehabilitation Act. (86 Fed. Reg. 61555, 61572.)
For more information on the CMS vaccine mandate and its impact on providers, please contact Felicia Y Sze or Samuel Chang.
(1) These states are: California, Colorado, Connecticut, Delaware, Florida, Hawaii, Illinois, Maine, Maryland, Massachusetts, Michigan, Minnesota, Nevada, New Jersey, New Mexico, New York, North Carolina, Oregon, Pennsylvania, Rhode Island, Tennessee, Vermont, Virginia, Washington and Wisconsin. This mandate also extends to the District of Columbia and U.S. territories.

Comments